Sapian predicts targets of Paracetamol (Acetaminophen)

Many of the drugs we consume every day still hold uncertainties. The Kantify Behind the Pill series shares novel insights and hypotheses about the untapped potential or the misunderstood side effects of drugs. The Behind the Pill analyses rely on the predictions of Kantify’s AI technology, Sapian™, a target and small molecule discovery platform.
In order to discover the hidden side of known molecules, Kantify has used Sapian™ to perform an in silico analysis of how drugs behave in the body. For this exercise, Sapian™ has analyzed no less than 78,000 drugs and predicted how they impact each of the known proteins in our body, also known as the proteome. This large-scale exercise has resulted in more than 1.4 billion predicted interactions—a groundbreaking achievement in AI-driven drug discovery. To share these findings with the scientific community, we regularly share our AI predictions for well-known drugs.
In this third article of the Behind the Pill series, we turn our focus to Paracetamol, one of the most used drugs worldwide. Our goal is to explore its complex mechanisms of action and uncover unexpected links to various health conditions. Paracetamol is an old drug that was first commercialized in the 1950’s. While Paracetamol is known for its role in reducing pain and fever, there have been reports (sometimes contested) of various biological effects. These include a potential role in the development of mental disorders, more precisely the onset of neurodegenerative diseases such as Parkinson’s or Alzheimer’s disease. Nevertheless, despite significant efforts and research, the mechanisms through which Paracetamol may induce these diverse effects have largely remained disputed.
In this work, we present the predictions generated by Sapian™ regarding proteins that bind to Paracetamol. Sapian™ has identified that Paracetamol may interact with over 291 proteins in the human body, which could help explain Paracetamol’s biological effects. Amongst these proteins, the AI has identified groups linked to pain relief (endocannabinoid system), oxidative stress (cytochromes, peroxidases, carbonic anhydrases) or neurological effects (neurotransmitters, glutathione peroxidase, and many more). Through the example of Paracetamol, Kantify demonstrates that AI can generate new hypotheses for many unknown mechanisms of drugs, potentially leading to new uses of existing drugs, and the development of targeted therapies.
A Peek Behind the Pill
Through its Behind the Pill series, Kantify aims to shed light on the unknown actions of commonly used drugs. In our first and second article, we tackled Sildenafil (Viagra™) and Diazepam (Valium™), and open-sourced our predictions for these two drugs.
We are continuing our exploration with one of the most used drugs in the world: Paracetamol, also known as Acetaminophen or Tylenol. Paracetamol is widely used for its pain- and fever-relieving effects and is available as an over-the-counter drug in a majority of countries (Figure 1).
Figure 1. (left) Tylenol™ pills [1]. (right) Doliprane™ pills [2]
The importance and complexity of understanding Paracetamol’s mechanisms
Despite its popularity and over a century of use, the mechanism of action of Paracetamol is still not fully understood [3,4]. While Paracetamol is generally considered safe when used within therapeutic limits, its availability and narrow safety margin make it potentially more harmful than other over-the-counter painkillers. Notably, the toxic dose of Paracetamol is 10 times its therapeutic dose, whereas the toxic dose of Ibuprofen is 60 times a therapeutic dose [5].
The risks associated with Paracetamol poisoning are evident in epidemiological data. This single drug is implicated in approximately 6% of all poisoning cases worldwide (see Figure 2). In the United Kingdom, Paracetamol-related overdoses account for nearly half (44%) of all overdose cases [6]. Moreover, Paracetamol is among the most common over-the-counter analgesics involved in intentional overdoses [7].
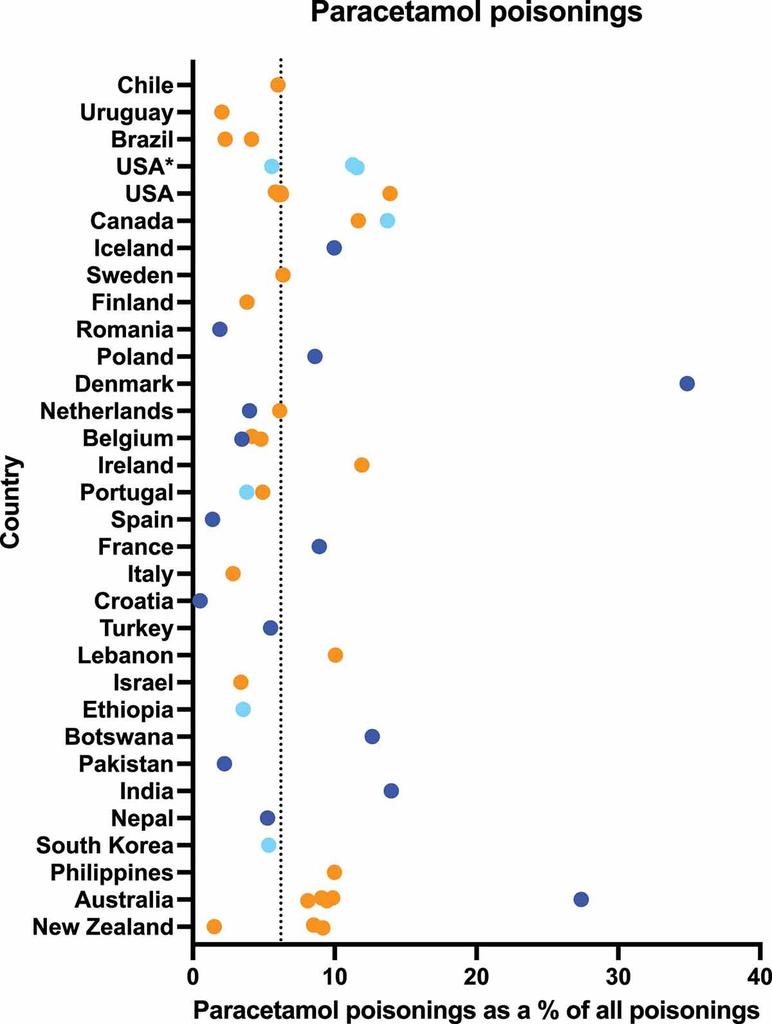
Figure 2. Percentage of poisonings involving Paracetamol compared to all poisonings, by country. Colors represent different data sources mentioned in the original paper[5]
A significant consequence of Paracetamol poisoning is hepatotoxicity, which occurs when excessive doses overwhelm the liver’s detoxification mechanisms, leading to the accumulation of toxic metabolites. Globally, Paracetamol-induced hepatotoxicity accounts for 56% of severe acute liver injuries and acute liver failures [5].
Beyond hepatotoxicity, emerging evidence raises concerns about potential neurotoxic effects of Paracetamol. For example, the role of Paracetamol in the development of neurodegenerative diseases, such as Parkinson’s and Alzheimer’s, remains an area of active debate [8–12]. This indicates that this drug has wide-ranging health effects through unknown and possibly novel mechanisms.
The mechanism of action (MoA) refers to how a drug produces its effects in the body—specifically, the biological pathways and targets it interacts with. There are several reasons why the MoA of Paracetamol remains a topic of debate today. To begin with, Paracetamol is an old drug. When it was first commercialized, advanced methods for understanding its MoA were unavailable; its use was based primarily on its observed clinical effects. Moreover, while Paracetamol is known for its pain-relieving and fever-reducing properties, the pathways involved in pain and fever are highly complex, involving many interrelated systems. Additionally, common NSAIDs (Non-Steroidal Anti-Inflammatory Drugs, such as Ibuprofen or Diclofenac) target cyclooxygenase (COX) proteins to alleviate pain and inflammation. However, Paracetamol binds weakly to some COX proteins and primarily exerts its effects in the brain, without significant peripheral anti-inflammatory activity. It is thought that Paracetamol may work through additional mechanisms, such as the endocannabinoid or serotonergic systems.
Given its popularity and wide-ranging health effects, advancing the understanding of Paracetamol’s MoA has important consequences. First and foremost, understanding these MoAs could lead to more selective usages of Paracetamol by not prescribing the drug to people at risk. In addition, finding new applications of Paracetamol could allow the treatment of more diseases. Lastly, the discovery of new MoAs underlying Paracetamol’s known side effects—such as hepatotoxicity, hypertension, serious cardiovascular events, and impaired development in early childhood [13–15]—could drive the development of first-in-class drugs. These new drugs could selectively harness the beneficial mechanisms of Paracetamol while minimizing or eliminating the associated risks, ultimately leading to safer treatment options for patients.
Using AI to uncover Paracetamol’s unknown interactions
To uncover Paracetamol’s likely MoAs, we combine predictions from our AI platform, Sapian™, with Paracetamol’s reported side effects and existing scientific knowledge about targets and pathways. Our goal is to investigate how Sapian™ correlates with existing knowledge and helps explain misunderstood side effects, as depicted in Figure 3 below. To achieve this, we apply a three-step methodology:
- Our AI creates a 2D atlas of the human proteome, allowing us to understand how Paracetamol’s targets are located through its eyes (Figure 7, at the end of the addendum).
- We add to the previous figure a heatmap generated by Sapian™ highlighting clusters of proteins that are most likely to explain Paracetamol’s wanted or unwanted effects.
- We seek tangible evidence that the identified clusters explain some of the effects of Paracetamol. This is done through an extensive literature review and an enrichment analysis using string-db.
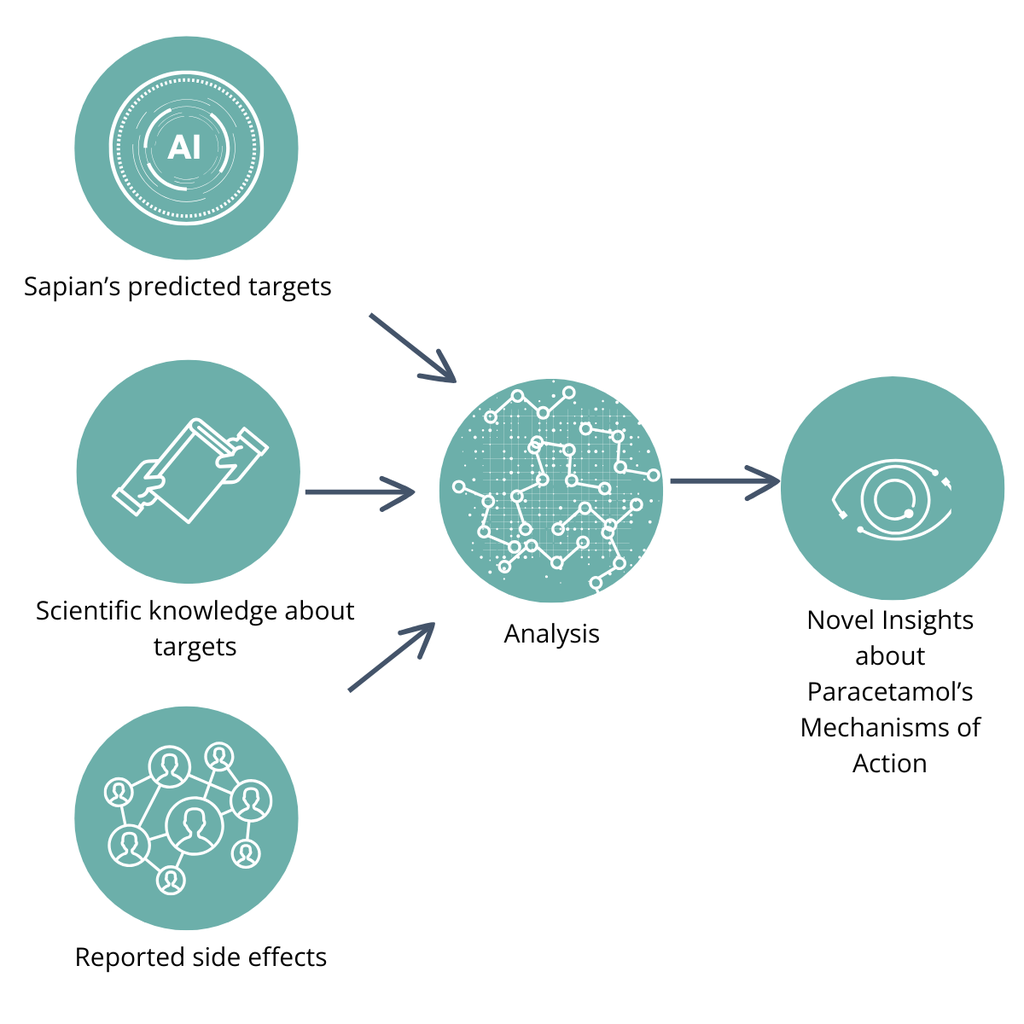
Figure 3. Our general approach to uncover Paracetamol’s MoAs.
The 2D atlas of Paracetamol’s targets as predicted by Sapian™ is represented by gray dots in Figure 4 below. This atlas displays the human proteome, mapped in a 2-dimensional space. Unlike a traditional geographical atlas, what matters in our map is proximity. More specifically, when proteins are close to one another, Sapian™ manages to see them as being similar—in terms of structure, drugability, biological function, or other important features.
In this map, warmer colors indicate a higher predicted likelihood that Paracetamol interacts with proteins in this space of the Atlas. This visual format not only highlights key areas with dense interactions but allows us to identify specific clusters of proteins that have not been extensively studied in relation to Paracetamol. These highlighted areas suggest new possibilities for research, encouraging us to delve into specific protein interactions that might reveal new uses for Paracetamol beyond its known applications.
Additionally, we have cross-referenced our predictions with existing scientific literature to verify if any of the predicted target interactions could be supported by already observed effects. This validation step is crucial as it anchors our predictions in real-world biological functions and helps refine our hypotheses.
In order to visually identify the polypharmacology of Paracetamol, one should look at the “warmer” zones, corresponding to protein families which Sapian™ predicts as the strongest biological targets of Paracetamol.
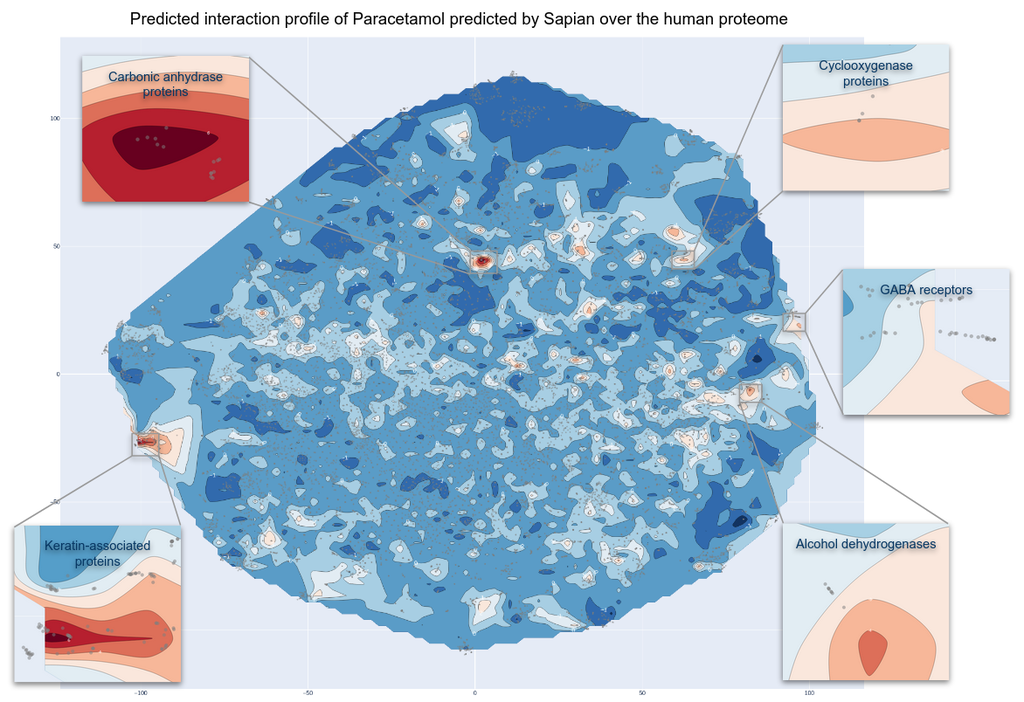
Figure 4. Kantify’s 2D Target Atlas for Paracetamol. Each dot represents a protein from the human proteome. Warm zones indicate a higher likelihood that Paracetamol interacts with these groups of families. Cutouts focus on 5 families. In this article, carbonic anhydrase proteins and GABA receptors will be detailed.
The analysis of Figure 4 shows that while the majority of the proteome exhibits negligible interaction probabilities with Paracetamol, there are potential interactions with around 291 proteins. This indicates that Paracetamol has a strong polypharmacological profile, although it is less pronounced compared to the two previously studied drugs in the Behind the Pill series (Sildenafil/Viagra™ and Diazepam/Valium™). This polypharmacology could explain its extensive range of side effects [16,17].
Now that we identified clusters of targets, we can further investigate their functions and associated pathways. Our next step is to assess, through literature, the potential interactions or links between these targets and Paracetamol. This will allow us to uncover potential new indications or MoAs.
Capturing Paracetamol’s broader impact
To further investigate the identified top targets potentially binding to Paracetamol, we conducted a network analysis using string-db. The top 291 targets were grouped using a clustering algorithm. This method organizes similar targets into groups based on their interactions, making it easier to identify patterns and relationships within the protein data. For each cluster, a pathway augmentation analysis allowed us to have deeper insights about the targets’ biological functions, pathways, or tissue expression. Combined with a comprehensive literature review, these findings allowed us to formulate hypotheses regarding Paracetamol’s potential novel modes of action, which are yet to be validated in wet labs.
Paracetamol induces oxidative stress which plays a role in liver injury and overall oxidative balance in the body
Oxidative stress is a well-documented consequence of Paracetamol metabolism, driven by the generation of reactive oxygen species. Between 5% and 10% of Paracetamol is metabolized into a highly toxic compound ( N -acetyl- p-benzoquinone imine, NAPQI), by cytochromes P450 in the liver [18], causing oxidative stress. Under physiological conditions, NAPQI is detoxified through conjugation with glutathione (GSH), resulting in the formation of a non-toxic metabolite that is subsequently excreted (Figure 5a). However, the liver’s GSH stores are limited, and once depleted, excess NAPQI reacts with liver proteins, leading to severe hepatotoxicity (see Figure 5b).
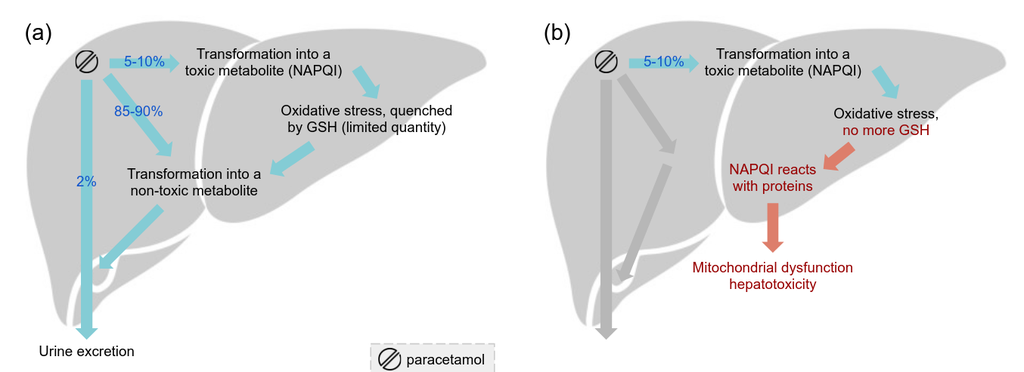
Figure 5. (a) Paracetamol’s metabolism in the liver (a) under physiological conditions and (b) under GSH stores depletion[18].
Sapian™ suggests that Paracetamol’s impact extends beyond cytochromes, involving additional targets potentially linked to oxidative stress and hepatotoxicity. Notably, quinone reductase 2 (NQO2) [19], myeloperoxidase [20], and several glutathione peroxidases [21,22] are pointed by Sapian™ as targets of Paracetamol. These targets are known to be implicated in oxidative stress pathways, supporting their potential involvement in Paracetamol-induced hepatotoxicity.
Five targets from two target families associated with oxidative stress in erythrocytes (red blood cells) were also highlighted by Sapian™. Erythrocytes are particularly sensitive due to their high exposure to oxygen and limited antioxidant capacity. Our pathway enrichment analysis identified targets involved in oxidation and oxygen/carbon dioxide exchange in erythrocytes, including carbonic anhydrases [23,24] (CA1, CA2, CA4) and heme oxygenases [25,26] (HMOX1, HMOX2). Paracetamol intake has been reported to influence oxidative stress markers in erythrocytes, potentially compromising their integrity and functionality [27–31].
Oxidative stress induced by Paracetamol may impact not only liver cells, contributing to hepatotoxicity, but also erythrocytes. Our predictions suggest its effect occurs through impairing their functionality as well as the overall oxidative balance in the body.
Paracetamol might play a role in the development of neurodegenerative diseases such as Alzheimer’s disease
Epidemiological evidence has suggested a link between regular Paracetamol intake and the onset of neurodegenerative diseases such as Alzheimer’s [8,9,12,32,33]. These studies point to a potential role for Paracetamol in accelerating neurodegeneration, warranting an investigation into the underlying mechanisms.
Oxidative stress
Oxidative stress, a mechanism already linked to Paracetamol-induced hepatotoxicity, also appears to play a significant role in neurodegenerative diseases [34] (Figure 6 below). Our analysis identified Glutathione Peroxidase [35] (GPx), a key antioxidant enzyme [36], as a predicted target of Paracetamol. GPx’s activity in combating ROS (Reactive Oxygen Species) in the liver may be mirrored in the brain, where oxidative stress could exacerbate neurodegeneration. Paracetamol’s predicted interaction with Casein Kinase 1 (CSNK1) further supports this hypothesis [37], as this protein phosphorylates tau, a hallmark of Alzheimer’s pathology, and contributes to amyloid-beta production [38,39].
Neurotransmission
Interference with synaptic transmission is another early feature of Alzheimer’s disease, often preceding its clinical onset [10,40–42]. Our analysis revealed that Paracetamol could potentially interact with several targets essential for neurotransmission, including gamma-aminobutyric acid (GABA), glycine, glutamate, acetylcholine, and serotonin receptors, as well as succinate-semialdehyde dehydrogenase (ALDH5A1). Long-term use of Paracetamol might subtly impair neuronal communication, contributing to the gradual accumulation of synaptic dysfunction that characterizes Alzheimer’s progression [10,43,44] (see Figure 6 below).
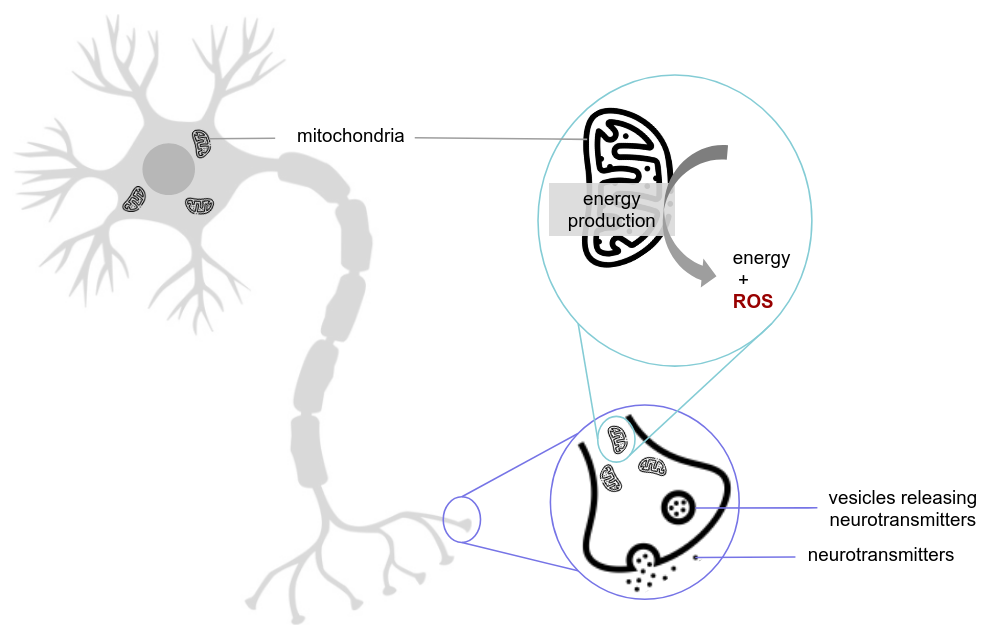
Figure 6. Simplified representation of a nerve cell. The first focus (purple) highlights a synapse, responsible for neuronal communication via neurotransmitter release. The second focus (blue) highlights a mitochondrion, essential for energy production. In Alzheimer’s disease, the number of mitochondria in nerve cells decreases, impairing energy production. Additionally, mitochondria generate reactive oxygen species (ROS) as a byproduct of their activity, contributing to oxidative stress[45,46].
Mitochondrial proteins
Our findings also highlight a cluster of mitochondrial proteins—COX1 (Cytochrome c oxidase subunit 1), NADH4 (NADH-ubiquinone oxidoreductase chain 4), NADH5 (NADH-ubiquinone oxidoreductase chain 5), and NDUFS7 (NADH dehydrogenase [ubiquinone] iron-sulfur protein 7, mitochondrial)—predicted to interact with Paracetamol. These proteins are essential for energy production, and their dysfunction is a hallmark of neurodegenerative diseases [47,48]. Given the brain’s high energy demands, mitochondrial dysfunction due to Paracetamol is also suspected to significantly contribute to the progression of Alzheimer’s disease [34,49–52].
Nitric oxide activity
The localization of Paracetamol-metabolizing enzymes in brain regions affected by Alzheimer’s further supports its potential role in neurodegeneration. Similar to the liver, toxic byproducts of Paracetamol metabolism form in the brain, potentially activating microglia—immune cells of the brain that detect damage and clear harmful substances. However, their activation can also trigger inflammation, including nitric oxide production, which may contribute to neurodegenerative processes [53]. Sapian™-predicted carbonic anhydrase proteins, known targets of Paracetamol and part of microglia, may partly modulate this nitric oxide activity. These proteins would therefore also play a role in Alzheimer’s [54,55].
A protective effect?
Interestingly, despite its potential risks in the brain, some studies suggest that Paracetamol might also exert protective effects. Evidence points to its interaction with the endocannabinoid system, which could mitigate neurodegeneration [56]. We can hypothesize that this effect may occur through predicted GABA receptors, previously identified as potential Paracetamol targets [57,58].
These findings suggest that Paracetamol may influence the onset and progression of Alzheimer’s disease through multiple pathways, including oxidative stress, tau phosphorylation, mitochondrial dysfunction, and impaired neuronal transmission. However, its potential neuroprotective effects through interactions with the endocannabinoid system underscores the complexity of its impact. Further research, for example about the role of GABA receptors, is needed to validate these interactions and explore their implications for neurodegenerative diseases.
A Call to Question and Expand Drug MoAs
Our findings reveal that beyond existing knowledge, drugs have potentially many more mechanisms. Using Sapian™, we are moving beyond the traditional single-target paradigms to reveal the unknown, yet crucial, complexity of polypharmacology. This AI-guided analysis of Paracetamol, one of the most consumed drugs worldwide, shows the need for this paradigm shift in drug discovery. Not only can we better explain the current usages of this molecule, but we also hope to launch new efforts to expand its use in treating more diseases.
Thank you for having read this 3rd issue of Behind the Pill.
Our Behind the Pill series started with Sildenafil (Viagra™) and [Diazepam (Valium™). The links to these papers can be found at the bottom of this page.
We invite clinicians, biologists, and the pharmaceutical community to provide feedback on our predictions.
For inquiries or suggestions on which drug to explore next, reach out to us at Kantify.
Abbreviations
- ADHD: Attention Deficit Hyperactivity Disorder
- AI: Artificial Intelligence
- ASD: Autism Spectrum Disorder
- GSH: Glutathione
- MoA: Mechanism of Action
- NAPQI: N -acetyl- p -benzoquinone imine
- NSAIDs: Non-Steroidal Anti-Inflammatory Drugs
- ROS: Reactive Oxygen Species
Disclaimer
The information provided in this article is for informational purposes only and is not intended as medical advice. This content should not be used to diagnose, treat, cure, or prevent any medical condition. Always consult a qualified healthcare provider for advice regarding any medical concerns or before starting or stopping any medication. The insights and findings discussed are based on research and are not a substitute for professional medical guidance.
Addendum
Are you researching the targets or off-targets of a small molecule that is in clinical trials or on the market? Get in touch.
Below is the list of predicted targets for Paracetamol, ordered by descending strength of signal.
Q9H0H5 Q9H0H5 P35219 Q8N1Q1 P59990 P07451 Q9NS85 P23280 P07451 Q8N1Q1 P35219 P23280 Q9BYT5 Q9NS85 Q9Y2D0 P00915 P43166 Q9BYR9 Q9BYU5 Q9BQ66 Q9ULX7 Q9BQS7 P59991 P35218 Q86XS5 Q9BYQ5 Q9BWU1 Q9BYR0 O75493 P59990 Q9BYR4 P60328 Q9BQS7 P00918 Q9BYQ9 P59991 Q52LG2 Q8IZU8 P00450 Q9BYR5 Q14314 P35218 P00450 Q8TAM1 Q9BYQ8 Q9BYR3 Q9BYQ7 P43166 Q3LI61 Q9Y2D0 Q3LI73 O43570 O43570 Q3LI73 Q8IUC0 Q8N6M5 O75493 Q6MZM0 Q3LHN2 Q9BYT5 Q9BYQ6 Q9BYR2 O43451 Q9BYR9 Q9BYU5 P22079 Q8IUC1 P35869 P00918 Q8IUC1 P00915 Q3LHN2 Q9BYQ7 Q6NZ63 A1A580 Q9BYR5 Q52LG2 P49336 P60328 Q9ULX7 Q9UHE8 P35869 Q3SYF9 Q3LI66 Q6ZW61 Q9BYQ9 Q13950 Q3LI76 P22079 Q9BYR0 Q9BYQ3 Q3LI64 P48730 Q3LI61 P07203 Q8IUC0 Q9BYQ8 Q9BYR3 Q8N6M5 Q9BYQ3 P05177 Q9UL01 A8MTY7 P04553 Q8IVQ6 P60329 Q9BQ66 O15537 Q9BYR4 Q15915 P49674 Q6MZM0 Q3LI77 Q9BYQ0 Q9BYQ2 P22748 Q86XS5 P15559 Q9BYQ0 P08294 Q8IUC2 Q14314 Q5T6V5 Q5T6V5 Q9BYQ5 Q3LI64 Q3LI77 Q9UNQ2 Q9BYQ4 P18283 P11766 P50440 Q9BYQ6 P16278 Q9NR21 Q9H993 Q3LI76 A8MTY7 Q8IUC2 Q3LI66 P08236 Q3SYF9 O43451 P04798 Q6P2C8 P14867 P15559 O75251 Q9BYR2 Q8WWA0 Q5VVY1 Q9BYP9 P02679 Q3LI70 Q9H5Q4 P04075 P10253 P22748 P14410 Q9BYQ2 O75884 Q9BYQ4 P53004 Q8WXA8 P05177 P07203 A1A580 P60891 P11678 P50440 Q8TET4 Q3LI70 P04798 Q9BYP9 P16278 P60372 Q8IUB9 Q93099 Q9BWU1 P08294 P21108 Q9BYR7 P35270 Q9Y2S2 P04553 Q9UM07 Q8IYX1 Q9NR21 Q8TAM1 Q8NCI6 Q5TA79 P50750 O43827 Q8IW92 P11766 P11908 Q9Y2S2 Q6TGC4 P60370 Q7Z4W3 P40126 O95409 P48729 P21108 P00395 P05093 P60329 P55789 P60014 Q6IQ16 Q9BYR7 Q8N752 P51580 P11678 A8MVA2 P60409 O15537 Q9UNQ2 P42262 Q08830 O75251 Q8TCX1 Q13057 Q15800 Q0VDG4 Q8NCR6 Q9BYR6 Q9BYR8 P18283 P55789 Q96GW9 Q16678 Q99598 P24941 Q9NTX5 P04406 P40126 A8MVA2 Q9UM07 P05164 P46952 P12955 P04075 P50135 Q9BYR8 P60370 P48730 Q9Y316 Q9NPJ1 P23109 P05164 P60372 Q687X5 Q9Y3B6 Q9BYR6 P09601 P34903 P51580 Q8NCI6 P14679 Q9Y256 Q8IW92 P11926 Q99598 Q8N752 P60014 Q6IQ16 Q8IZU8 Q3LI59 P28476 P40261 O75695 P08236 Q8WWU7 Q8N5Y8 P60409 Q3LI59 Q9H4Y5 P47869 Q9H773 A2VDF0 P18507 Q9UKN8 P51649 Q6PEX3 P04066 P14920 P30837 P05093 P49674 Q3SY46 P06493 P06744 Q16678 P0DSO2 Q3LI67 Q08830 O43280 P47870 O75083 Q9Y2V0 Q96MV1 Q9HCN8 Q9NX20 Q8N5T2 P08572 Q4G0Z9 Q9NTX5 Q13761 P0DSO2 O75695 Q8WXA8 P06865 P14679 Q5TA81 Q32M88 Q9NR97 P04406 Q6ZW61 O60674 Q9Y547 P11310 Q92626 Q9BXD5 Q8WW14 Q8N142 Q99928 Q04727 Q9UL01 A8MXZ3 Q6N063 Q9GZT4 Q01196 P42261 Q8IWZ5 O75083 Q16445 P06493 P23109 Q5TA82 Q9H0W9 P16083 Q9BXD5 P60891 P24941 Q70EL3 Q8N142 P49641 A2RUB1 Q96GW9 P30041 Q9NTJ4 Q6TGC4 Q96T66 P00338 Q9Y276 P31644 P14920 Q96IY4 Q13950 Q9ULW8 Q3LI72 Q93099 Q9GZT4 Q5VZ03 Q6UWY0 P02675 Q5TA76 Q96LM6 Q9HCI6 Q5TA77 O43827 Q8WW27 Q6YP21 P24046 O14960 A8MXZ3 P08686 Q14697 Q9HCN8 Q15631 Q8IUH4 Q5TA79 Q9NPJ1 Q5TA77 P23416 P30519 Q3SY46 Q8N1A6 Q96E35 Q9HCJ1 P49189 Q9Y276 Q0VDG4 Q9UFW8 Q8N8Y2 Q9BVC4 P00492 Q96C23 Q86X55 O14633 Q9H993 Q8N5Z0 P09601 Q00526 Q96FA3 P30041 C0HLU2 P36969 Q8WWA0 P40261 P50135 Q96IY4 Q5VZ03 P05166 Q9BV86 Q13057 Q9HD40 Q9UK59 P26599 Q9UNU6 Q9H5Q4 P60371 Q9BRA2 O94788 Q16517 P11908 Q9H773 Q14055 Q15631 Q5TA76 Q6PEX3 Q7RTV0 Q96C23 P0CG40 Q8N8R7 P36544 Q16853 O15498 O43895 Q99675 P08686 P06865 Q9BVC4 P00338 P06733 P03915 Q6YP21 P03905 P26368 O14933
Authors
Rubal Ravinder, Nicolas Maignan, David Papazian, Maxime Georges, Jack Dawe, Caio Hudson de Souza, Ségolène Martin, Nik Subramanian
About Paracetamol
Paracetamol is a drug originally developed and commercialized under the name Panadol by Sterling-Winthrop Co. With its patent now expired, it is widely marketed under many names. Below are some of the most well-known brand names for Paracetamol: Tylenol®: developed by Johnson & Johnson Inc. Doliprane®: developed by Sanofi Efferalgan®: developed by UPSA
Sources
- Martin, C. Excessive Use of Tylenol & OTC Painkillers Causes Severe Health Issues. Martin & Helms, P.C. https://martinhelms.com/blog/excessive-use-of-tylenol-and-otc-painkillers-is-causing-severe-health-problems/ (2022).
- Paracétamol : tout ce que vous devez savoir sur son utilisation et ses risques. www.rtl.fr https://www.rtl.fr/actu/sante/paracetamol-tout-ce-que-vous-devez-savoir-sur-son-utilisation-et-ses-risques-7900446861 (2024).
- Ayoub, S. S. Paracetamol (acetaminophen): A familiar drug with an unexplained mechanism of action. Temp. Multidiscip. Biomed. J. 8, 351 (2021).
- How Does Acetaminophen Work? | School of Medicine. https://medicine.tufts.edu/news-events/news/how-does-acetaminophen-work.
- Chidiac, A. S., Buckley, N. A., Noghrehchi, F. & Cairns, R. Paracetamol (acetaminophen) overdose and hepatotoxicity: mechanism, treatment, prevention measures, and estimates of burden of disease. Expert Opin. Drug Metab. Toxicol. (2023).
- Paracetamol self-poisoning: Epidemiological study of trends and patient characteristics from the multicentre study of self-harm in England. J. Affect. Disord. 276, 699–706 (2020).
- Shoib, S. et al. Over‐the‐counter drug use in suicidal/self‐harm behavior: Scoping review. Health Sci. Rep. 5, e662 (2022).
- Nilsson, S. E. et al. Does aspirin protect against Alzheimer’s dementia? A study in a Swedish population-based sample aged > or =80 years. Eur. J. Clin. Pharmacol. 59, 313–319 (2003).
- Breitner, J. C. Epidemiologic clues to the causes and routes to prevention of Alzheimer disease. J. Neural Transm. Suppl. 59, 251–254 (2000).
- Jones, G. R. N. The Alzheimer pandemic: is paracetamol to blame? Inflamm. Allergy Drug Targets 13, 2–14 (2014).
- Jones, G. R. Causes of Alzheimer’s disease: paracetamol (acetaminophen) today? Amphetamines tomorrow? Med. Hypotheses 56, 121–123 (2001).
- Zhang, Y. et al. Association of regular use of ibuprofen and paracetamol, genetic susceptibility, and new-onset dementia in the older population. Gen. Hosp. Psychiatry 84, 226–233 (2023).
- Jóźwiak-Bebenista, M. & Nowak, J. Z. Paracetamol: mechanism of action, applications and safety concern. Acta Pol. Pharm. 71, 11–23 (2014).
- McCrae, J. C., Morrison, E. E., MacIntyre, I. M., Dear, J. W. & Webb, D. J. Long‐term adverse effects of paracetamol – a review. Br. J. Clin. Pharmacol. 84, 2218–2230 (2018).
- Brune, K., Renner, B. & Tiegs, G. Acetaminophen/paracetamol: A history of errors, failures and false decisions. Eur. J. Pain 19, 953–965 (2015).
- Acetaminophen Side Effects: Common, Severe, Long Term. Drugs.com https://www.drugs.com/sfx/acetaminophen-side-effects.html.
- Acetaminophen: MedlinePlus Drug Information. https://medlineplus.gov/druginfo/meds/a681004.html.
- Moles, A., Torres, S., Baulies, A., Garcia-Ruiz, C. & Fernandez-Checa, J. C. Mitochondrial–Lysosomal Axis in Acetaminophen Hepatotoxicity. Front. Pharmacol. 9, (2018).
- Miettinen, T. P. & Björklund, M. NQO2 Is a Reactive Oxygen Species Generating Off-Target for Acetaminophen. Mol. Pharm. 11, 4395–4404 (2014).
- Du, K., Ramachandran, A. & Jaeschke, H. Oxidative stress during acetaminophen hepatotoxicity: Sources, pathophysiological role and therapeutic potential. Redox Biol. 10, 148–156 (2016).
- Lubos, E., Loscalzo, J. & Handy, D. E. Glutathione Peroxidase-1 in Health and Disease: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid. Redox Signal. 15, 1957–1997 (2011).
- Pei, J., Pan, X., Wei, G. & Hua, Y. Research progress of glutathione peroxidase family (GPX) in redoxidation. Front. Pharmacol. 14, (2023).
- Di Fiore, A., Monti, D. M., Scaloni, A., De Simone, G. & Monti, S. M. Protective Role of Carbonic Anhydrases III and VII in Cellular Defense Mechanisms upon Redox Unbalance. Oxid. Med. Cell. Longev. 2018, 2018306 (2018).
- Gurel, Z. & Sheibani, N. Potential of Carbonic Anhydrase Inhibitors in the Treatment of Oxidative Stress and Diabetes. in The Carbonic Anhydrases: Current and Emerging Therapeutic Targets (eds. Chegwidden, W. R. & Carter, N. D.) 121–146 (Springer International Publishing, Cham, 2021). doi:10.1007/978-3-030-79511-5_6.
- Basuroy, S. et al. HO-2 provides endogenous protection against oxidative stress and apoptosis caused by TNF-alpha in cerebral vascular endothelial cells. Am. J. Physiol. Cell Physiol. 291, C897-908 (2006).
- Chiang, S.-K., Chen, S.-E. & Chang, L.-C. The Role of HO-1 and Its Crosstalk with Oxidative Stress in Cancer Cell Survival. Cells 10, 2401 (2021).
- Orhan, H. & Sahin, G. In vitro effects of NSAIDS and paracetamol on oxidative stress-related parameters of human erythrocytes. Exp. Toxicol. Pathol. Off. J. Ges. Toxikol. Pathol. 53, 133–140 (2001).
- Randle, L. E. et al. alpha(1)-Adrenoceptor antagonists prevent paracetamol-induced hepatotoxicity in mice. Br. J. Pharmacol. 153, 820–830 (2008).
- Yuan, J., Wei, H., Jin, W., Yang, X. & Wang, E. Kinetic study of paracetamol on prolidase activity in erythrocytes by capillary electrophoresis with Ru(bpy)(3) (2+) electrochemiluminescence detection. Electrophoresis 27, 4047–4051 (2006).
- Kozer, E. et al. Glutathione, glutathione-dependent enzymes and antioxidant status in erythrocytes from children treated with high-dose paracetamol. Br. J. Clin. Pharmacol. 55, 234–240 (2003).
- Kerchberger, V. E. & Ware, L. B. The Role of Circulating Cell-Free Hemoglobin in Sepsis-Associated Acute Kidney Injury. Semin. Nephrol. 40, 148–159 (2020).
- Lövheim, H., Karlsson, S. & Gustafson, Y. The use of central nervous system drugs and analgesics among very old people with and without dementia. Pharmacoepidemiol. Drug Saf. 17, 912–918 (2008).
- Hamina, A. et al. Differences in analgesic use in community-dwelling persons with and without Alzheimer’s disease. Eur. J. Pain Lond. Engl. 21, 658–667 (2017).
- Misrani, A., Tabassum, S. & Yang, L. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease. Front. Aging Neurosci. 13, (2021).
- Tomečková, V., Gajová, A., Veliká, B., Saxunová, L. & Hertelyová, Z. Prooxidative and fluorescence properties of paracetamol during interactions with mitochondria. J. Spectrosc. 25, 174237 (2011).
- Zhang, J. et al. The functions of glutathione peroxidase in ROS homeostasis and fruiting body development in Hypsizygus marmoreus. Appl. Microbiol. Biotechnol. 104, 10555–10570 (2020).
- Melov, S. et al. Mitochondrial Oxidative Stress Causes Hyperphosphorylation of Tau. PLOS ONE 2, e536 (2007).
- Chen, C. et al. Up-regulation of casein kinase 1ε is involved in tau pathogenesis in Alzheimer’s disease. Sci. Rep. 7, 13478 (2017).
- Yasojima, K., Kuret, J., DeMaggio, A. J., McGeer, E. & McGeer, P. L. Casein kinase 1 delta mRNA is upregulated in Alzheimer disease brain. Brain Res. 865, 116–120 (2000).
- Meftah, S. & Gan, J. Alzheimer’s disease as a synaptopathy: Evidence for dysfunction of synapses during disease progression. Front. Synaptic Neurosci. 15, (2023).
- Li, K. et al. Synaptic Dysfunction in Alzheimer’s Disease: Aβ, Tau, and Epigenetic Alterations. Mol. Neurobiol. 55, 3021–3032 (2018).
- Wu, M. et al. The role of pathological tau in synaptic dysfunction in Alzheimer’s diseases. Transl. Neurodegener. 10, 45 (2021).
- Alzheimer’s stages: How the disease progresses. Mayo Clinic https://www.mayoclinic.org/diseases-conditions/alzheimers-disease/in-depth/alzheimers-stages/art-20048448.
- Parnetti, L., Chipi, E., Salvadori, N., D’Andrea, K. & Eusebi, P. Prevalence and risk of progression of preclinical Alzheimer’s disease stages: a systematic review and meta-analysis. Alzheimers Res. Ther. 11, 7 (2019).
- Moreira, P. I., Carvalho, C., Zhu, X., Smith, M. A. & Perry, G. Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim. Biophys. Acta BBA - Mol. Basis Dis. 1802, 2–10 (2010).
- Reddy, P. H. A Critical Assessment of Research on Neurotransmitters in Alzheimer’s Disease. J. Alzheimers Dis. JAD 57, 969–974 (2017).
- Picca, A. et al. Mitochondrial Dysfunction, Protein Misfolding and Neuroinflammation in Parkinson’s Disease: Roads to Biomarker Discovery. Biomolecules 11, 1508 (2021).
- Chen, C. et al. Parkinson’s disease neurons exhibit alterations in mitochondrial quality control proteins. Npj Park. Dis. 9, 1–14 (2023).
- Jiang, J. et al. Increased mitochondrial ROS formation by acetaminophen in human hepatic cells is associated with gene expression changes suggesting disruption of the mitochondrial electron transport chain. Toxicol. Lett. 234, 139–150 (2015).
- Jaeschke, H., Duan, L., Nguyen, N. & Ramachandran, A. Mitochondrial Damage and Biogenesis in Acetaminophen-induced Liver Injury. Liver Res. 3, 150 (2019).
- Mohammadi, A., Kazemi, S., Molayousefian, I., Pirzadeh, M. & Moghadamnia, A. A. Galangin Nanoparticles Protect Acetaminophen-Induced Liver Injury: A Biochemical and Histopathological Approach. Evid.-Based Complement. Altern. Med. ECAM 2022, 4619064 (2022).
- Yu, L. et al. Single-cell RNA sequencing reveals the dynamics of hepatic non-parenchymal cells in autoprotection against acetaminophen-induced hepatotoxicity. J. Pharm. Anal. 13, 926–941 (2023).
- Yuste, J. E., Tarragon, E., Campuzano, C. M. & Ros-Bernal, F. Implications of glial nitric oxide in neurodegenerative diseases. Front. Cell. Neurosci. 9, (2015).
- Giovannuzzi, S. et al. Dual Inhibitors of Brain Carbonic Anhydrases and Monoamine Oxidase-B Efficiently Protect against Amyloid-β-Induced Neuronal Toxicity, Oxidative Stress, and Mitochondrial Dysfunction. J. Med. Chem. 67, 4170–4193 (2024).
- Lemon, N., Canepa, E., Ilies, M. A. & Fossati, S. Carbonic Anhydrases as Potential Targets Against Neurovascular Unit Dysfunction in Alzheimer’s Disease and Stroke. Front. Aging Neurosci. 13, (2021).
- Labib, A. Y. et al. Mechanistic insights into the protective effect of paracetamol against rotenone-induced Parkinson’s disease in rats: Possible role of endocannabinoid system modulation. Int. Immunopharmacol. 94, 107431 (2021).
- Blecharz-Klin, K. et al. Paracetamol impairs the profile of amino acids in the rat brain. Environ. Toxicol. Pharmacol. 37, 95–102 (2014).
- Kim, M. et al. Expression Levels of GABA-A Receptor Subunit Alpha 3, Gabra3 and Lipoprotein Lipase, Lpl Are Associated with the Susceptibility to Acetaminophen-Induced Hepatotoxicity. Biomol. Ther. 25, 112–121 (2017).